
Exhibit 99.2

NASDAQ: UNCY Renazorb: BE Study Update
Renazorb Background 2 • Renazorb • Novel lanthanum - based phosphate binder • In development for the treatment of hyperphosphatemia in CKD patients on dialysis • Regulatory Pathway • Agreement with the FDA on acceptability of 505(b)(2) pathway for registration • Followed FDA BE study guidance for the development of the protocol • Because of low systemic absorption of lanthanum, BE study is based on a pharmacodynamic - based endpoint rather than a pharmacokinetic - based endpoint • Agreement with the FDA on the study design, dose and endpoints for the BE study
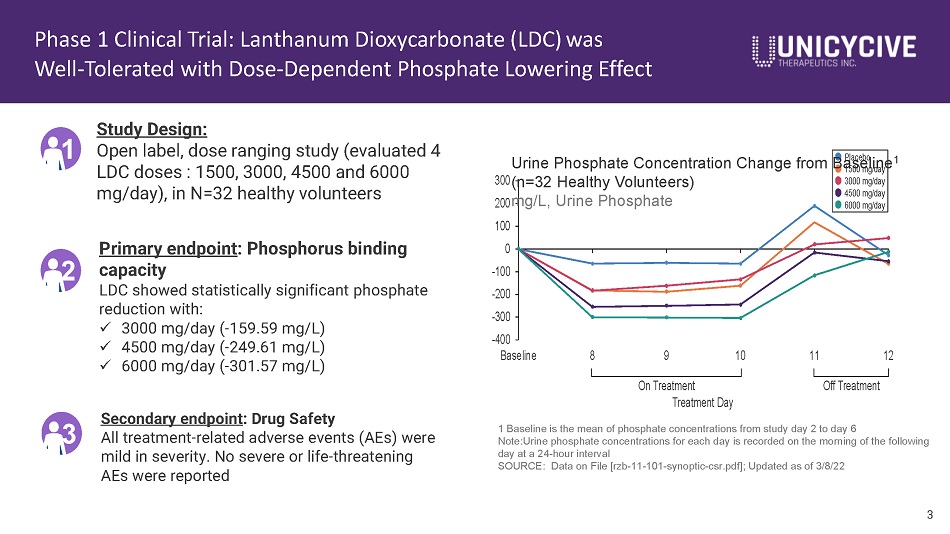
Phase 1 Clinical Trial: Lanthanum Dioxycarbonate (LDC) was Well - Tolerated with Dose - Dependent Phosphate Lowering Effect 1 Study Design: Open label, dose ranging study (evaluated 4 LDC doses : 1500, 3000, 4500 and 6000 mg/day), in N=32 healthy volunteers Primary endpoint : Phosphorus binding capacity LDC showed statistically significant phosphate reduction with: x 3000 mg/day ( - 159.59 mg/L) x 4500 mg/day ( - 249.61 mg/L) x 6000 mg/day ( - 301.57 mg/L) Secondary endpoint : Drug Safety All treatment - related adverse events (AEs) were mild in severity. No severe or life - threatening AEs were reported 2 3 100 0 - 100 - 200 - 300 - 400 Baseline 8 9 10 11 12 On Treatment Treatment Day Off Treatment 1500 mg/day 3000 mg/day 4500 mg/day 6000 mg/day 3 Urine Phosphate Concentration Change from B a P l a s c e e b o li n e 1 300 (n=32 Healthy Volunteers) 200 mg/L, Urine Phosphate 1 Baseline is the mean of phosphate concentrations from study day 2 to day 6 Note:Urine phosphate concentrations for each day is recorded on the morning of the following day at a 24 - hour interval SOURCE: Data on File [rzb - 11 - 101 - synoptic - csr.pdf]; Updated as of 3/8/22
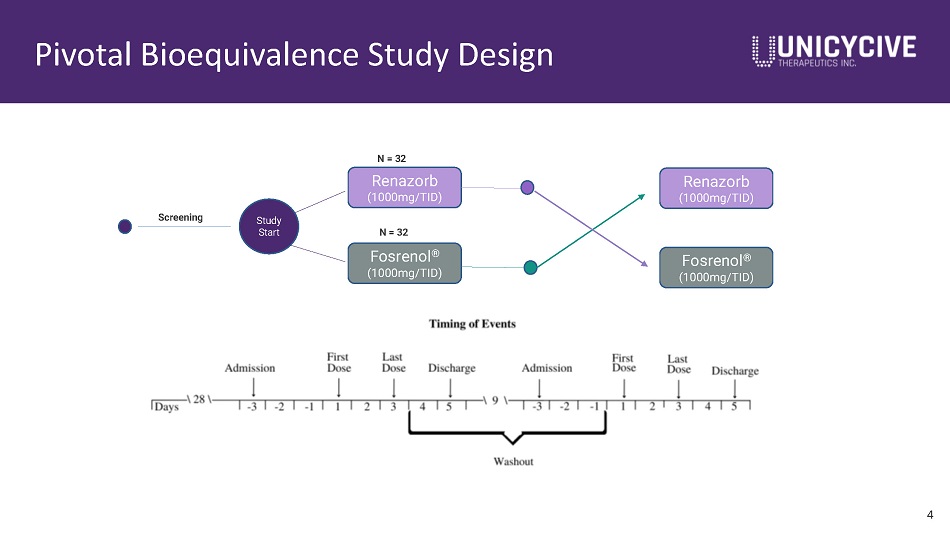
Pivotal Bioequivalence Study Design Study Start Fosrenol ® (1000mg/TID) N = 32 Renazorb (1000mg/TID) Screening N = 32 Renazorb (1000mg/TID) Fosrenol ® (1000mg/TID) 4

Renazorb Bioequivalence Study in Healthy Volunteers 5 Objectives Primary Objective To demonstrate pharmacodynamic (PD) equivalence of orally administered Renazorb 1000 mg TID to orally administered Fosrenol 1000 mg TID in healthy subjects Secondary Objective To compare the safety and tolerability of Renazorb versus Fosrenol in healthy subjects Endpoints Primary Endpoint Least squares (LS) mean change in urinary phosphate excretion (in mg/day) from baseline to the evaluation period. Secondary Endpoint Safety endpoints include incidence, frequency, and severity of treatment - emergent adverse events (TEAEs), including serious adverse events and adverse events (AEs) resulting in treatment discontinuation. • Baseline is defined as the approximately 48 - hour urine collection period starting on Day - 2 and ending on Day 1. • Evaluation period is defined as the approximately 72 - hour urine collection period starting on Day 1 and ending on Day 4.
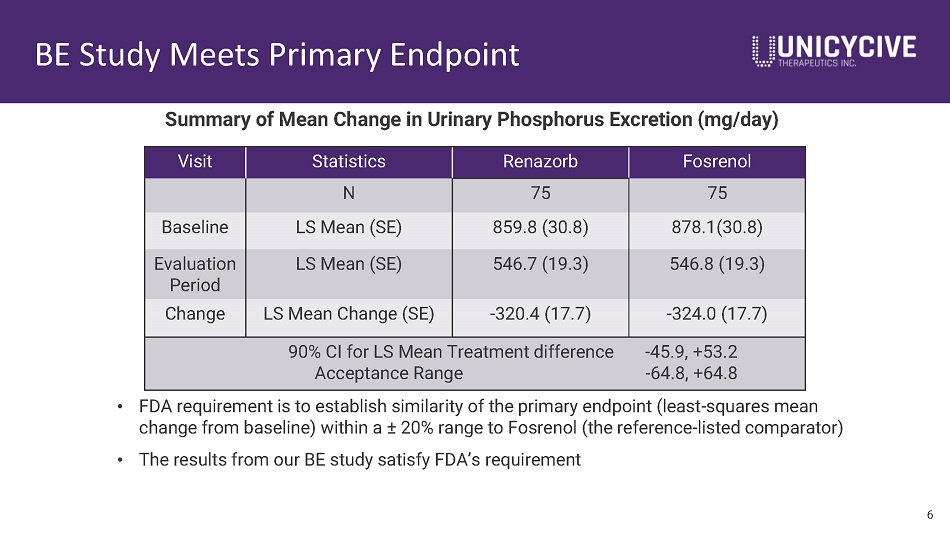
BE Study Meets Primary Endpoint 6 Visit Statistics Renazorb Fosrenol N 75 75 Baseline LS Mean (SE) 859.8 (30.8) 878.1(30.8) Evaluation Period LS Mean (SE) 546.7 (19.3) 546.8 (19.3) Change LS Mean Change (SE) - 320.4 (17.7) - 324.0 (17.7) 90% CI for LS Mean Treatment difference - 45.9, +53.2 Acceptance Range - 64.8, +64.8 Summary of Mean Change in Urinary Phosphorus Excretion (mg/day) • FDA requirement is to establish similarity of the primary endpoint (least - squares mean change from baseline) within a “ 20% range to Fosrenol (the reference - listed comparator) • The results from our BE study satisfy FDA’s requirement
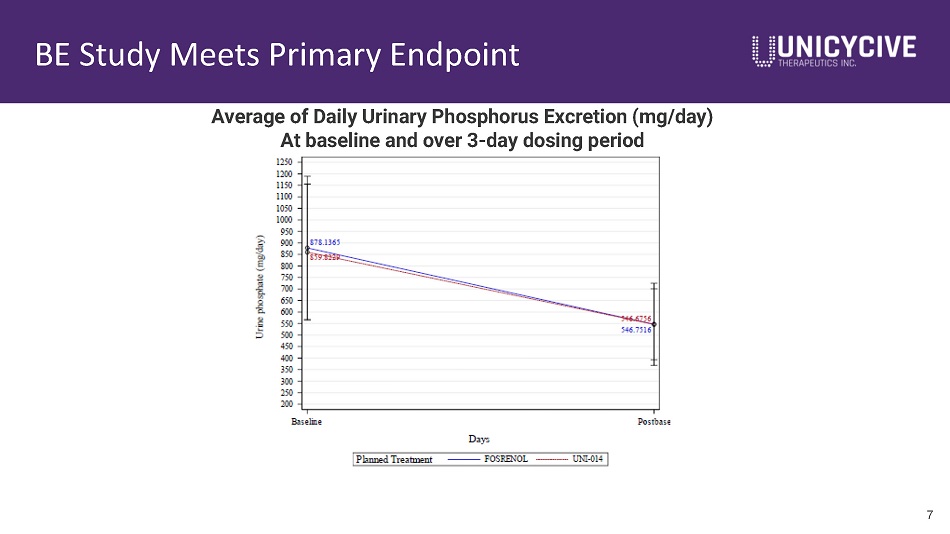
BE Study Meets Primary Endpoint 7 Average of Daily Urinary Phosphorus Excretion (mg/day) At baseline and over 3 - day dosing period

Safety Comparable Between Renazorb and Fosrenol 8 • Treatment emergent adverse events were comparable between the Renazorb and Fosrenol groups • No subjects in the study experienced a serious adverse event (requiring hospitalization or withdrawal from the study) • There were no deaths in the study
Summary 9 • Efficacy • Study met the primary endpoint • Bioequivalence established based on the pharmacodynamic endpoint • Reference interval was defined as ц 20% of the LS mean of the primary PD variable for lanthanum carbonate • The 90% CI of the primary PD variable for Renazorb was completely contained within the reference interval • This approach is based on the statistical method for evaluation of bioequivalence described in various regulatory guidelines [FDA BE Study Guidance 2001; EMA Guidelines for Bioequivalence 2010 and FDA BE Study Guidance 2022] • Safety • Comparable to Fosrenol
Successful Renazorb BE Study Outcome Establishes Bridge to Fosrenol Clinical Data 10 • By successfully demonstrating pharmacodynamic bioequivalence between Renazorb and Fosrenol (505(b)(2) reference - listed drug), a bridge to Fosrenol’s already approved clinical efficacy and safety data set is established • 5 RCTs in CKD patients on dialysis (both hemodialysis and peritoneal dialysis) • 2,325 total patients studied • Clearly documented clinical efficacy • ~ 2.0 mg/dL reduction in serum phosphorus (compared to ~ 0.70 mg/dL for ARDX’s tenapanor) • Established safety and tolerability • Incidence of adverse events (AEs) in pivotal phase 3 trial similar to placebo • Above clinical profile becomes Renazorb product label and can be used in promotion of our product

Renazorb Value Proposition Renvela ® sevelamer carbonate 800mg 3 tablets per day Volume : 2.3 cm 3 Renazorb Œ lanthanum dioxycarbonate 1,000mg 9 tablets per day Volume : 9.7 cm 3 Renazorb offers patients a 4 - fold reduction in daily pill burden compared to Renvela — currently the most prescribed phosphate binder 11 vs Through its proprietary nanoparticle technology, Unicycive has harnessed the phosphate binding potency of lanthanum to reduce the number and size of pills that patients must take to control hyperphosphatemia
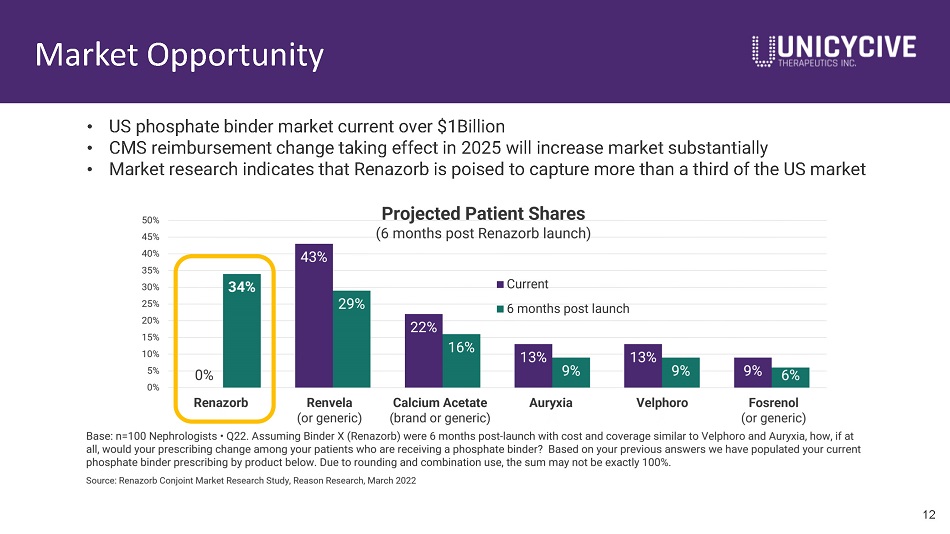
Market Opportunity • US phosphate binder market current over $1Billion • CMS reimbursement change taking effect in 2025 will increase market substantially • Market research indicates that Renazorb is poised to capture more than a third of the US market 12